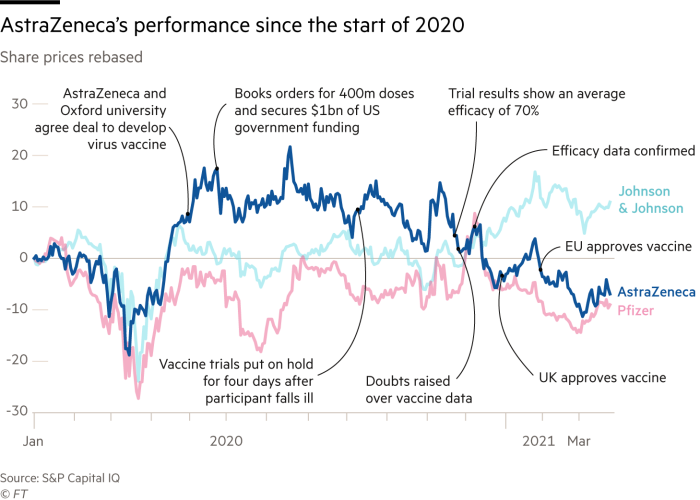
Last summer, eight years into his term as chief executive of AstraZeneca, Pascal Soriot was riding high. The share price was up 200 per cent on his watch. The company’s pipeline had been transformed since the Parisian saw off a takeover bid from Pfizer in 2014. And the UK-Swedish pharma group was working with Oxford university on the world’s leading vaccine candidate for Covid-19.
Since then the vaccine has been injected into millions of arms and saved thousands of lives. Yet Soriot and AstraZeneca are enduring constant criticism for their distribution and data difficulties. The stock is down by more than a fifth since July, lagging behind a dozen larger rivals. There are even rumblings of discontent with the superstar CEO.
This has been another week of drama. On Sunday, Soriot’s friend José Baselga, AstraZeneca’s head of oncology R&D, died in Spain. On Monday, AstraZeneca unveiled data in the US showing high efficacy levels for its Covid-19 vaccine, allowing the company to bask briefly in the acclaim its executives feel they deserve.
But early on Tuesday morning, the data and safety monitoring board in the US abruptly disputed the findings, with the clear implication that AstraZeneca had inflated the performance by excluding the most up-to-date data.
Even as Soriot took charge of the latest crisis, one person close to him described the usually unflappable 61-year-old Frenchman as “a bit frazzled . . . He is very good at planning and seeing and dealing with everything. But this came completely out of the blue and he hates it when something he hasn’t forecast happens.”
AstraZeneca on Thursday released additional data about the US trials, which showed only a slight reduction in efficacy at preventing symptomatic Covid-19 infection — from 79 to 76 per cent. The success at preventing serious illness and death remained at 100 per cent.
But even this delayed vindication offered little respite. In the middle of the US crisis, another problem erupted in Europe. EU officials suggested on Wednesday that the company had “stockpiled” 29m doses at a factory in Italy, fuelling anger that vaccines made in the EU were being shipped overseas even as EU countries suffered severe delays. In reality, most of the doses were destined for the EU, the company said.
Questions are now mounting about whether the very dash and drive that had enthralled pharma investors have led to missteps, as Soriot mobilised AstraZeneca to deliver a vaccine on a grand scale and at cost for the duration of the pandemic.
One former senior employee said AstraZeneca “felt like they are martyr-heroes coming to try to save the world . . . but getting stuck in this battle between the UK and the EU, mostly because the EU has a Brexit grudge”.
Guts but no glory
It took guts to take on a challenge of this size: making a commitment to producing more vaccines than any other player, despite little vaccine experience. But the company’s tendency to rely on Soriot’s “gut instinct” can also cause it problems, one person close to the company suggested. “He doesn’t have a lot of people pushing back on anything he says,” she said.
Even on the board there is concern that “everything has to go through Pascal”, according to one person familiar with internal discussions, who added that some directors were querying the strength of the people around the chief executive.
A person close to the company rejected that, pointing to key roles played by Mene Pangalos, head of biopharma R&D, Ruud Dobber, head of the biopharma business, and Pam Cheng, head of global operations and IT.
Perhaps significantly, for a company that has suffered public relations problems, there is nobody on the senior executive team with communications as a core part of their responsibilities, a difference from rivals such as Pfizer or GlaxoSmithKline.
One insider said: “There is a general sense of the company being buffeted on the reputational issue and just not being able to get on top of it.”
In this week’s example, the company had wanted to release promptly the findings from its large US trial, aware they could help lay to rest damaging questions about its safety and benefits, but had neglected to agree the press release in advance with the independent monitoring board.
Few lessons seemed to have been learned from an early data release in November, which caused confusion and controversy when three separate efficacy levels were included. Only later did it emerge that one dosing regime had been due to an initial error and had not been tested on people over the age of 55.
The lack of transparency has riled scientists as well as officials. Anthony Fauci, the veteran director of the US National Institute of Allergy and Infectious Diseases, this week said he had never seen a data safety monitoring board react so vehemently to a public release.
“When something goes wrong with a product, it’s seldom the case that it’s only a communications problem, but that appears to be the case with the AstraZeneca vaccine,” said John Doorley, associate professor of strategic communications at Elon University and former corporate communications director at Merck.
“The available data show they’ve got a great vaccine and they’re offering it in a way that’s civically responsible, but it looks like the rush to satisfy legitimate communication pressures from the stock market and public health officials has led to some missteps.”
The scientists behind the creation of the Oxford vaccine are frustrated that its merits are not resounding as loudly as they should, according to a person familiar with the matter, who suggested it was creating some tensions with AstraZeneca. The Oxford team want a co-ordinated effort to trumpet its advantages, particularly in the US, and to ensure it is not seen simply as a “British” vaccine, the person said.
Oxford university said it was “working closely with AstraZeneca, to make the most of their expertise in drug development and strong global networks. This is allowing us to move fast and start protecting people around the world . . . ”
The former senior employee said she was not surprised that AstraZeneca had run into problems with the board that oversaw the US clinical trial, even though no other vaccine makers had. She said AstraZeneca had been disappointed that the vaccine was not as effective as the mRNA vaccines from BioNTech/Pfizer and Moderna and was keen to make its efficacy rate look as good as possible.
“They are willing to go into the grey zone,” she said.
A person close to the company said this was completely incorrect.
Over-promise, under-deliver
While what one person described as Soriot’s “optimism” is credited for the company’s extraordinary revival into one of the best-performing global drug stocks in recent years, the same quality was also being viewed as “a slight weakness”, said another person who cited the way in which he had “over-promised” on the volumes and timescale for vaccine production in Europe.
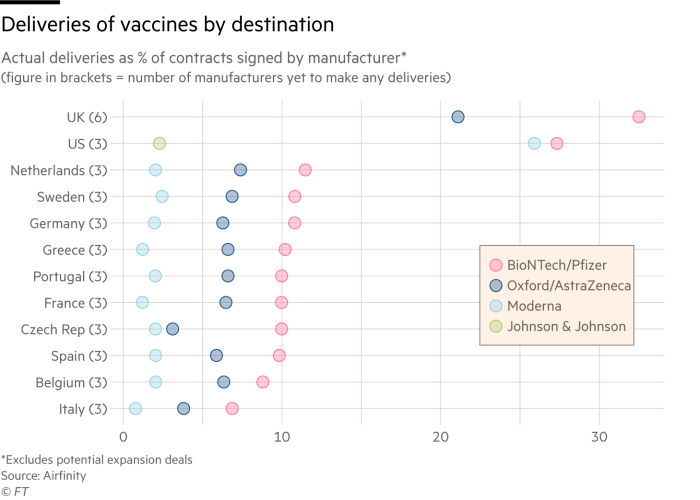
When Soriot met EU officials in February, he promised to deliver a combined total of 150m doses in the second quarter. That target was recently cut to 70m. It is on course to miss the first quarter’s target of 30m, which had already been slashed from an original upper limit of 120m.
Soriot said half of these doses would come from China and the US, according to people with knowledge of the interaction — but neither country has so far exported doses and the US has some export restrictions.
The person close to the company said it had indicated that it would try to increase deliveries by sourcing from its international network — from India rather than China — but that it was not guaranteed. As soon as it had become clear that this would not be possible in the near term, it had informed the European Commission.
Other questions centre on an interview Soriot gave to European media in January, in which he said the vaccine active ingredients for Europe were “essentially produced in two plants, one in the Netherlands, one in Belgium”. Yet the Dutch factory, run by the subcontractor Halix, has not delivered a single dose to the EU because it has yet to receive regulatory authorisation there.
Many European officials no longer bother to hide their fury with AstraZeneca, which they portray as a main reason why the EU immunisation drive badly lags behind those in the UK and US.
Philippe Lamberts, co-leader of the Green group in the European Parliament, said commission officials had been “telling me for months now that AstraZeneca treats us like a piece of shit”.
“We learn some stuff from the press about commitments. This is not the way you deal with a customer,” he told the FT. “It would seem AstraZeneca has a major problem of organisational culture. It’s a culture of unreliability.”
Suggesting there could be lasting damage to AstraZeneca’s business with the EU, he added: “I wouldn’t want to be in the shoes of Pascal Soriot. Because they are shooting themselves in the foot permanently.”
Thierry Breton, EU internal market commissioner, told the FT he was in “constant contact” with Soriot but had “not always received consistent explanations” from AstraZeneca about supply problems. “The company needs to explain to us why there is such a difference in treatment between the UK and Europe,” Breton said.

In contrast, rival vaccine maker Johnson & Johnson has also faced some production problems, but EU officials praise it for being cautious. “A good merchant is never, never over-promising,” said one official.
Both Soriot and Leif Johansson, the company’s chair, have flinched at finding themselves caught in such a public spotlight, one insider said. “They are both very private people. They just want the whole thing to go away.”
Investors keep the faith
So far, no heads have rolled. Those with the power to push Soriot out — the shareholders and the board — are keeping the faith. Mike Fox, a fund manager and head of sustainable investing at Royal London Asset Management, a top-20 shareholder with a £900m stake, praised the company and Soriot as “heroic”.
“It is important to understand it is, right now, the maximum point of heat and tension,” he said. “It would be much better to think about what the reputation will be in two years’ time and our view is it will become the dominant vaccine.”
Two other top-30 investors told the FT they supported the CEO.
Yet amid the febrile atmosphere this week, questions are being asked among some board members about how Soriot operates. Internally recent events have contributed to a sense of what one source described as “lost momentum . . . the share price has gone from £90 to £70”. The company’s £94bn market cap was still “extraordinary” but, however unfairly, “it’s now seen as a slight disappointment” against last summer’s £122bn peak.
The same person said there had even been the odd muttering at a high level in the company about whether the CEO, who is a naturalised Australian citizen, has spent enough time in the UK, although most senior colleagues regarded his work rate, from whatever part of the world and in whatever timezone, as extraordinary. The person close to the company said Soriot was working “European business hours” and was planning to return to Europe from Australia as soon as the lockdown ended.

Peter Bach, director of the Center for Health Policy and Outcomes at Memorial Sloan-Kettering, called for Soriot to resign. He argued AstraZeneca’s stumbles could be responsible for a public health “catastrophe” as tens of millions of people could not take the vaccine because of the company’s mistakes.
He said that if the board did not fire him, it was because “they think he’s the best chief executive for the shareholders, whether or not he’s the best chief executive for the public health crisis”.
Hans van Ees, a professor of corporate governance at the University of Groningen, said the real question for the board was whether AstraZeneca’s slip-ups were foreseeable.
“The vaccine rollout is clearly being managed from the C-suite,” he said, “and eventually the CEO will have to own some of these possible errors of judgment if they do terminal damage to AstraZeneca’s reputation.”
Reporting by Sarah Neville in London, Hannah Kuchler in New York, Michael Peel and Sam Fleming in Brussels, Donato Paolo Mancini in Rome and Attracta Mooney and Oliver Barnes in London
Business - Latest - Google News
March 26, 2021 at 01:48AM
https://ift.tt/3feQRBT
Disputes leave AstraZeneca boss with chronic vaccine headache - Financial Times
Business - Latest - Google News
https://ift.tt/2Rx7A4Y
Bagikan Berita Ini














0 Response to "Disputes leave AstraZeneca boss with chronic vaccine headache - Financial Times"
Post a Comment